Developing a biosimilar is a complex process. Biosimilars, like all biologics, are produced through an intricate, multistep process, using living cells. However, the cell line and manufacturing process of the reference product are proprietary and belong to the original manufacturer. Biosimilarity can be established through evaluation of the biosimilar in various experiments and testing including active comparator clinical trials, in comparison to the reference biologic.1-4
Manufacturing biologics, including biosimilars, is more nuanced than manufacturing a small molecule medicine. The complexities of manufacturing innovator biologics also apply to biosimilar medicines. But biosimilar development and manufacturing also has its own set of intricacies.5 The video below helps explain.
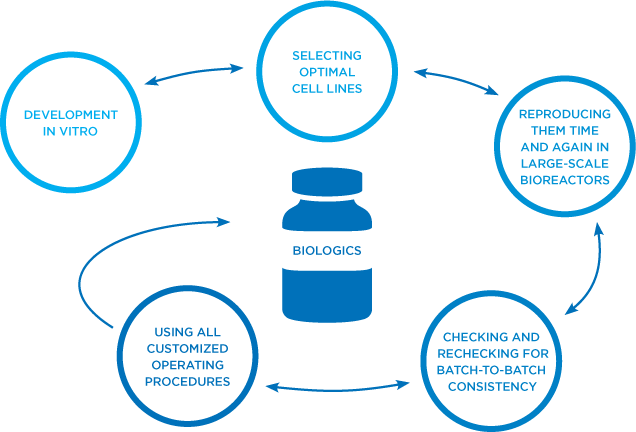
Our biologic medicines are manufactured using living cells engineered to produce therapeutic proteins in large quantities. These cells are very sensitive to conditions produced during their synthesis and handling and a series of culturing and purification steps are required to produce a consistent, quality active ingredient.6

There is a strong relationship between the manufacturing process and characteristics of a biologic medicine. Even small changes in manufacturing can impact biologic activity and, by extension, affect overall efficacy, safety, or immunogenicity.3 It is critical that appropriate safeguards be established to protect patients. Click the link below to read more about the process of manufacturing safe and effective biologics.