CLEAR
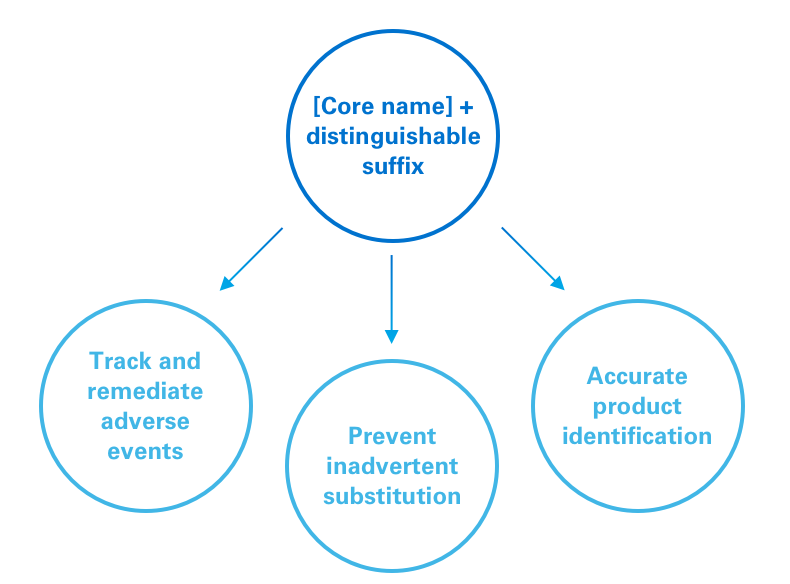
Most regulatory bodies require specific naming and labeling practices for biosimilars. This is so patients can know what they are taking, doctors will know what they are prescribing and what pharmacists are dispensing, and regulatory bodies can accurately track adverse events and other real-world data.
It’s important to emphasize that naming and labeling requirements vary among regulatory authorities. For example, in the FDA has the following requirements: