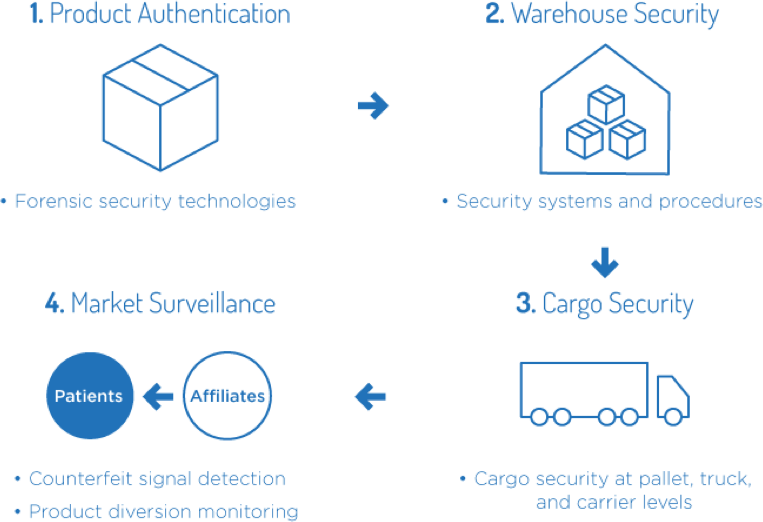
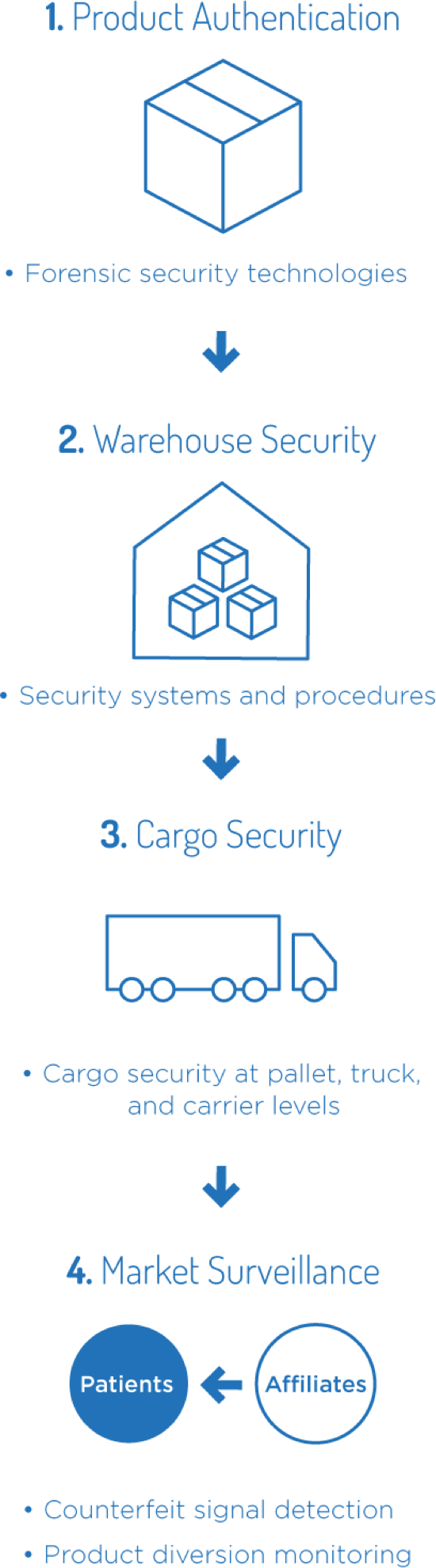
Amgen implements product security programs to help minimize the
risk of supply disruption for patients5
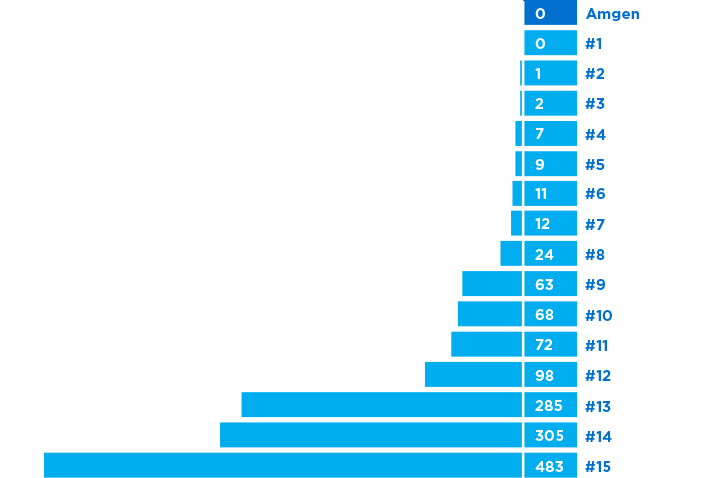
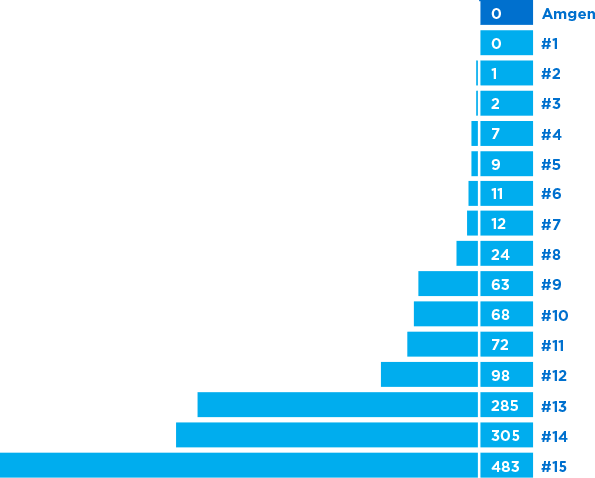
Total number of drug shortages 2007-20176,*
References: 1. Data on file, Amgen; [Quality Control Checks] 2018. 2. Mysler E, Pineda C, Horiuchi T, et al. Clinical and regulatory perspectives on biosimilar therapies and intended copies of biologics in rheumatology. Rheumatol Int. 2016;36:613-625. 3. US Government Accountability Office. Drug shortages: public health threat continues, despite efforts to help ensure product availability. GAO-14-194. www.gao.gov/assets/670/660785.pdf. Accessed October 29, 2019. 4. Ebel T, George K, Larsen E, Shah K, Ungerman D. Building new strengths in the healthcare supply chain. McKinsey & Company. www.health.economictimes.indiatimes.com/web/files/retail_files/reports/data_file-Building-New-Strenghts-In-Healthcare-Supply-Chain-1421845643.pdf. Accessed October 29, 2019. 5. Mica A, Mutomba M, Green L. Steps to ensure adequate supply of biological medicines: considerations for the healthcare provider. GaBI J. 2013;2:136-143. 6. Data on file, Amgen; 2018.