The first biosimilar was approved by the European Union in 2006. Since then, the number of approved biosimilars has increased worldwide, and they are expected to play an increasingly important role in healthcare.1,2
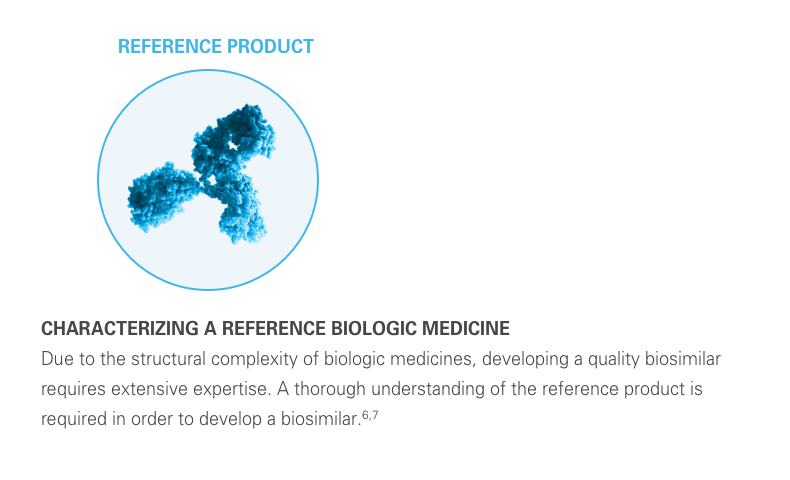
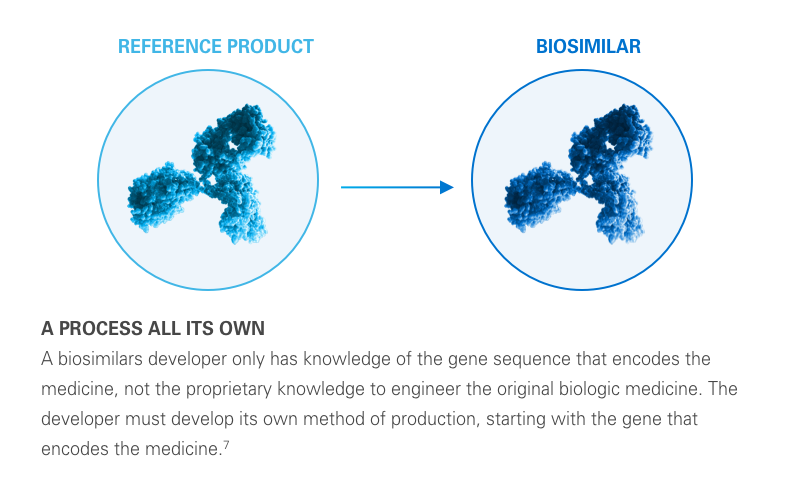
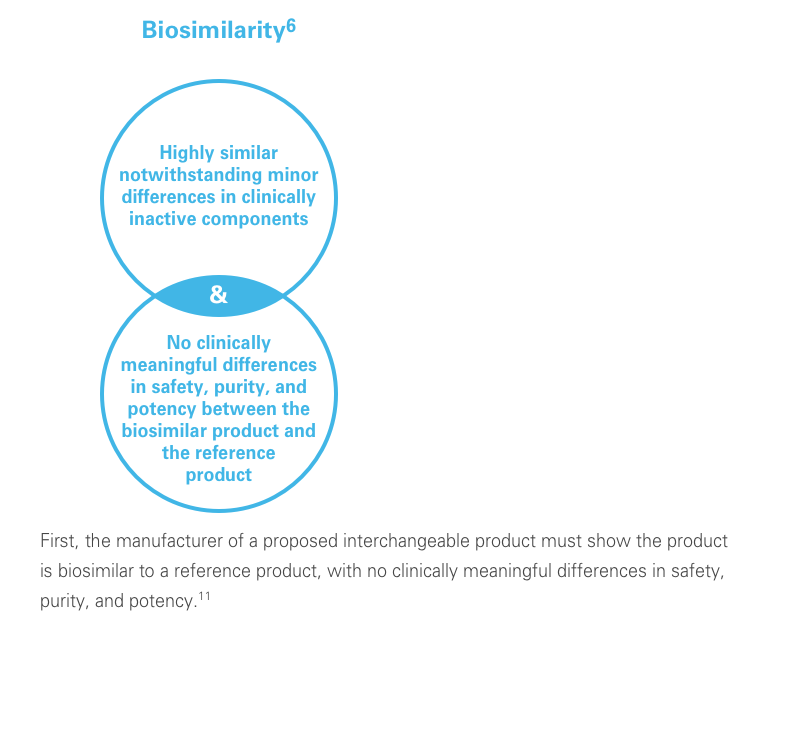
A biosimilar is a biological product designed to be both highly similar to, and have “no clinically meaningful differences” from, its reference product. It’s important to remember that biosimilars are not generic drugs. Biosimilars are up to 1,000 times the size of small molecule generic drugs, and are far more structurally complex.5-8
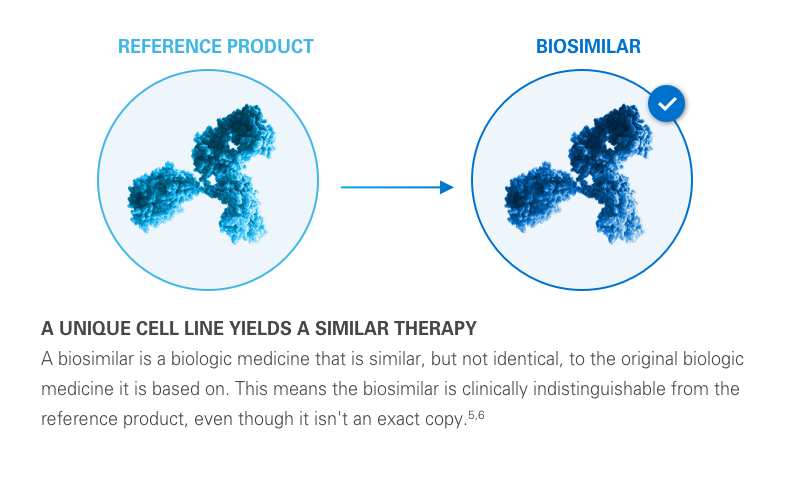
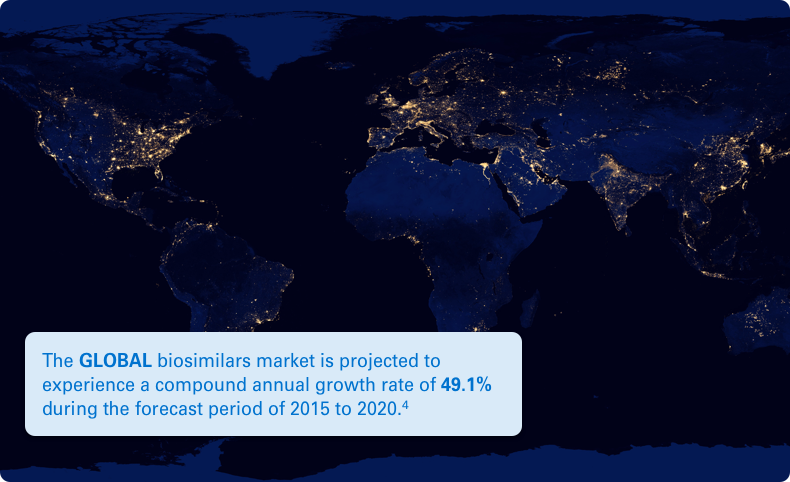
Typically, there is a lower cost of development for a biosimilar than that of its reference biologic. This lower cost can help reduce the financial impact of medications on the healthcare system. Plus, it can help provide more treatment options for potentially deadly diseases like cancer.2,3
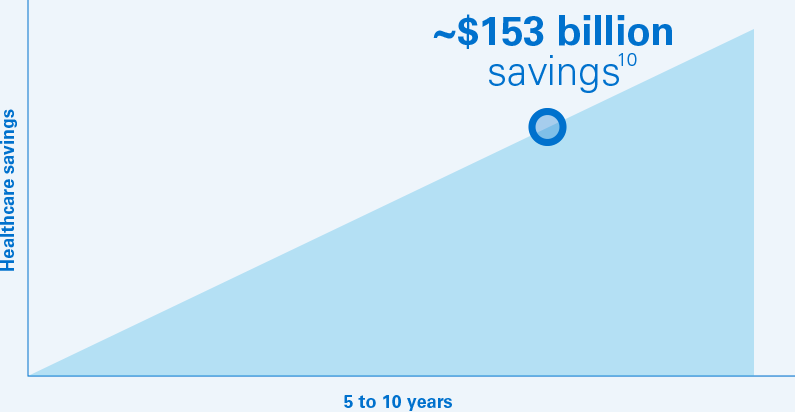
The projected annual savings to the US healthcare system could climb to $60 billion in 2023 for a 5‑year total of $153 billion.10
The clinical testing requirements for biosimilars differ from those of the reference product. Still, biosimilars manufacturers must submit robust data, such as extensive analytical studies, plus nonclinical and clinical evidence. This totality of evidence is required before a biosimilar is approved for the market.6
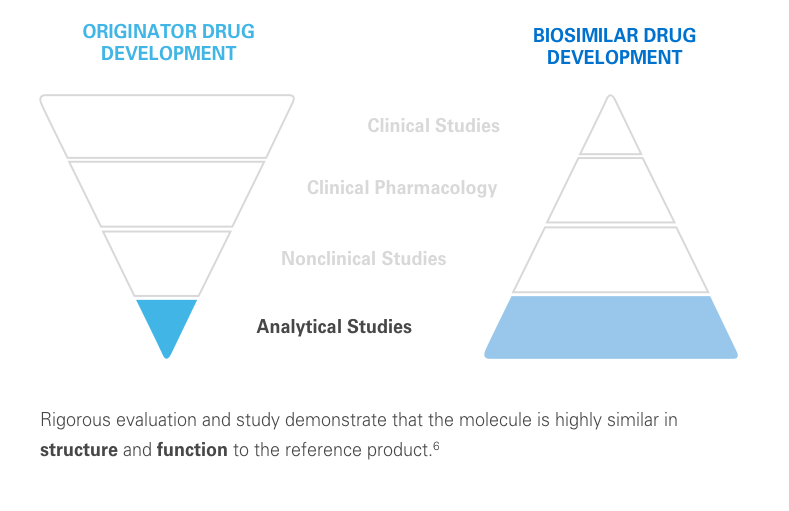
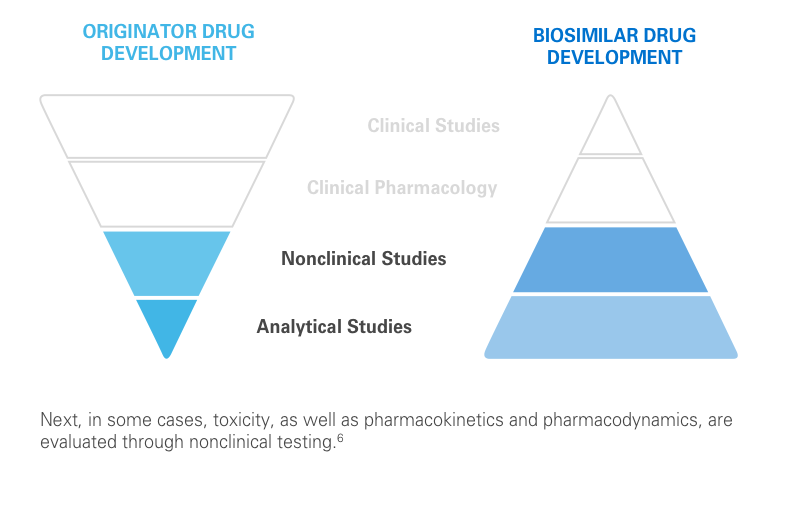
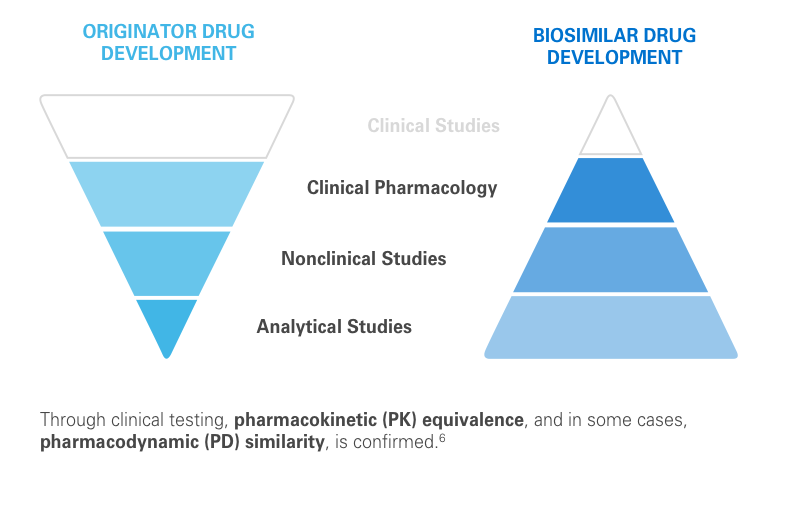
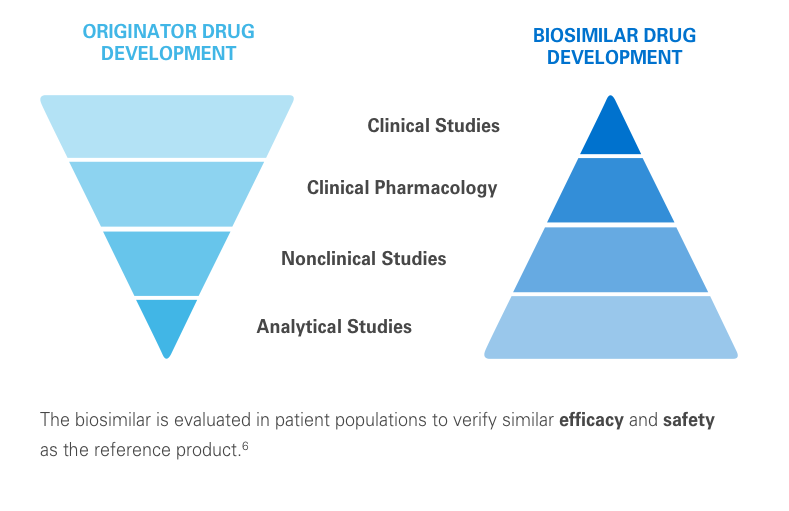
Regulatory agencies review the totality of evidence to determine whether the biosimilar can be approved for some or all of the indications of the reference product.11
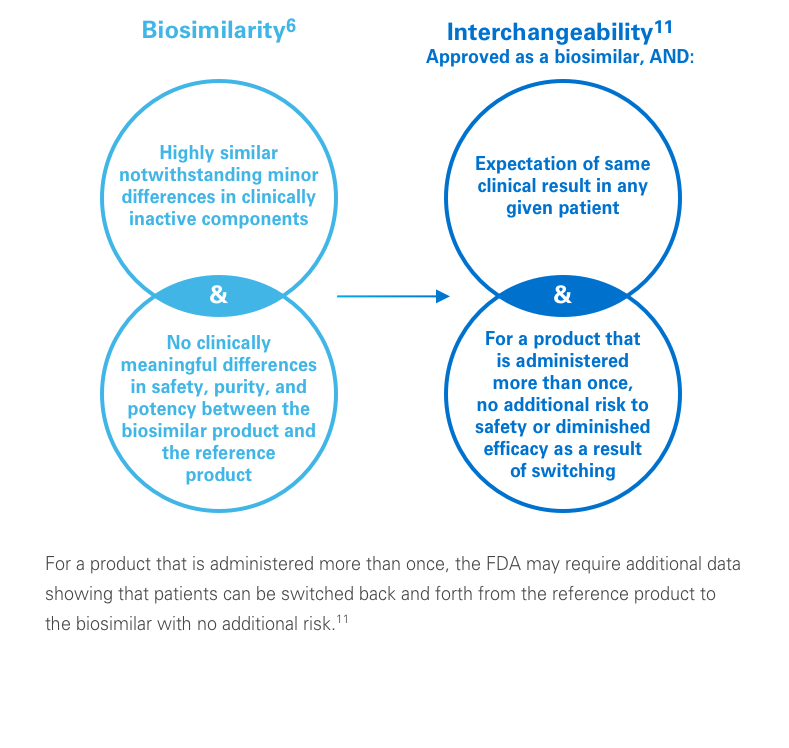
While most generic drugs can be substituted by a pharmacist for the branded reference product, the same is not necessarily true for biosimilars. Only biosimilars that undergo additional analyses to support an FDA designation of “interchangeable” can be substituted by a pharmacist for a prescribed reference biologic, where consistent with state pharmacy laws.12
References: 1. European Medicines Agency. Biosimilars in the EU: information guide for healthcare professionals. www.ema.europa.eu/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf. Accessed October 29, 2019. 2. US Food and Drug Administration. Biosimilars action plan: balancing innovation and competition. www.fda.gov/downloads/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/ucm613761.pdf. Accessed October 29, 2019. 3. The Biosimilars Council. Biosimilars in the United States: providing more patients greater access to lifesaving medicines. www.biosimilarscouncil.org/wp-content/uploads/2017/09/Biosimilars-Council-Patient-Access-Study-090917.pdf. Accessed October 29, 2019. 4. Allied Market Research. Biosimilars market (follow-on-biologics) by types (human growth hormone, erythropoietin, monoclonal antibodies, insulin, interferon, granulocyte-colony stimulating factor, peptide) and application (blood disorders, oncology diseases, growth hormone deficiencies)-global opportunity analysis and industry forecast, 2014-2020. www.alliedmarketresearch.com/global-biosimilars-market. Accessed October 29, 2019. 5. European Medicines Agency. Questions and answers on biosimilar medicines (similar biological medicinal products). www.medicinesforeurope.com/wpcontent/uploads/2016/03/WC500020062.pdf. Accessed October 29, 2019. 6. US Food and Drug Administration. Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product. www.fda.gov/downloads/drugs/guidances/ucm291128.pdf. Accessed October 29, 2019. 7. Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol. 2008;19:411-419. 8. Lee JF, Litten JB, Grampp G. Comparability and biosimilarity: considerations for the healthcare provider. Curr Med Res Opin. 2012;28:1053-1058. 9. Blauvelt A, Cohen AD, Puig L, Vender R, van der Walt J, Wu JJ. Biosimilars for psoriasis: preclinical analytical assessment to determine similarity. Br J Dermatol. 2016;174:282-286. 10. The Center For Biosimilars. Biosimilars market is ripe for cost savings. www.centerforbiosimilars.com/contributor/chad-pettit/2019/06/biosimilars-market-is-ripe-for-cost-savings. Accessed October 29, 2019. 11. US Food and Drug Association. Biosimilar and interchangeable products. www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products. Accessed October 29, 2019. 12. US Food and Drug Administration. Considerations in demonstrating interchangeability with a reference product. www.fda.gov.downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm537135.pdf. Accessed October 29, 2019.