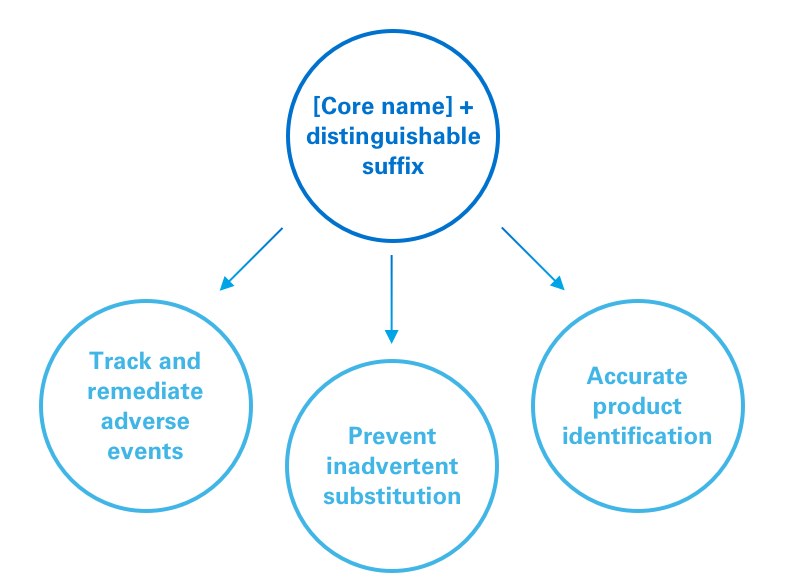
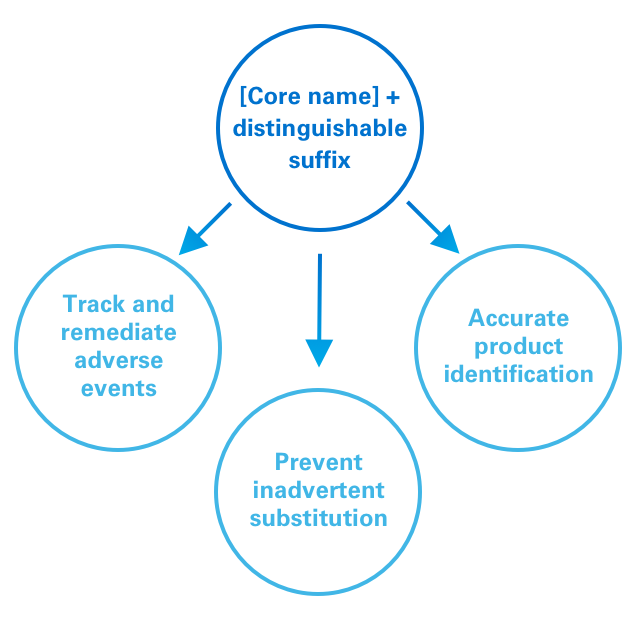
The FDA has implemented a policy requiring distinguishable suffixes as part of the nonproprietary name of biologic medicines.1
For reference biologics, the FDA recommends using a “core name” that is designated by the United States Pharmacopeial Convention with a 4-letter suffix. For a biosimilar, the core name will be the same as the reference biologic, but with a different 4-letter suffix.1
This policy is designed to:1
1. Help accurately track adverse events;
2. Facilitate appropriate targeting of remedial action, eg, to a specific product vs a whole class;
3. Minimize risk of inadvertent substitution; and
4. Promote accurate identification of products by pharmacists and providers.
Reference: 1. US Food and Drug Administration. Guidance for industry: nonproprietary naming of biological products. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/UCM459987.pdf. Accessed October 29, 2019.