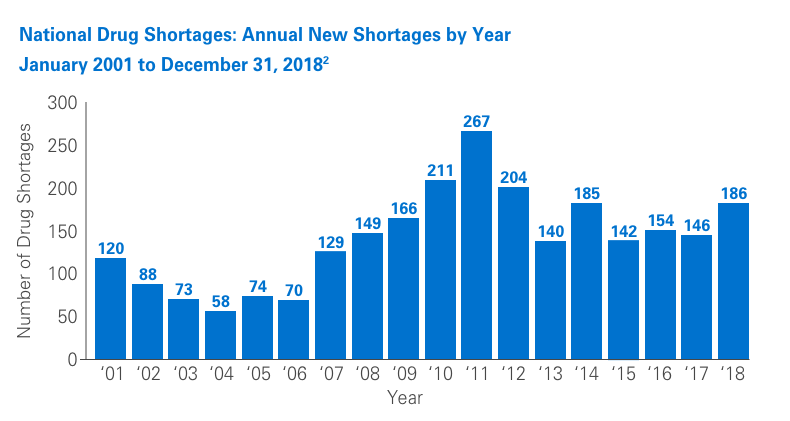
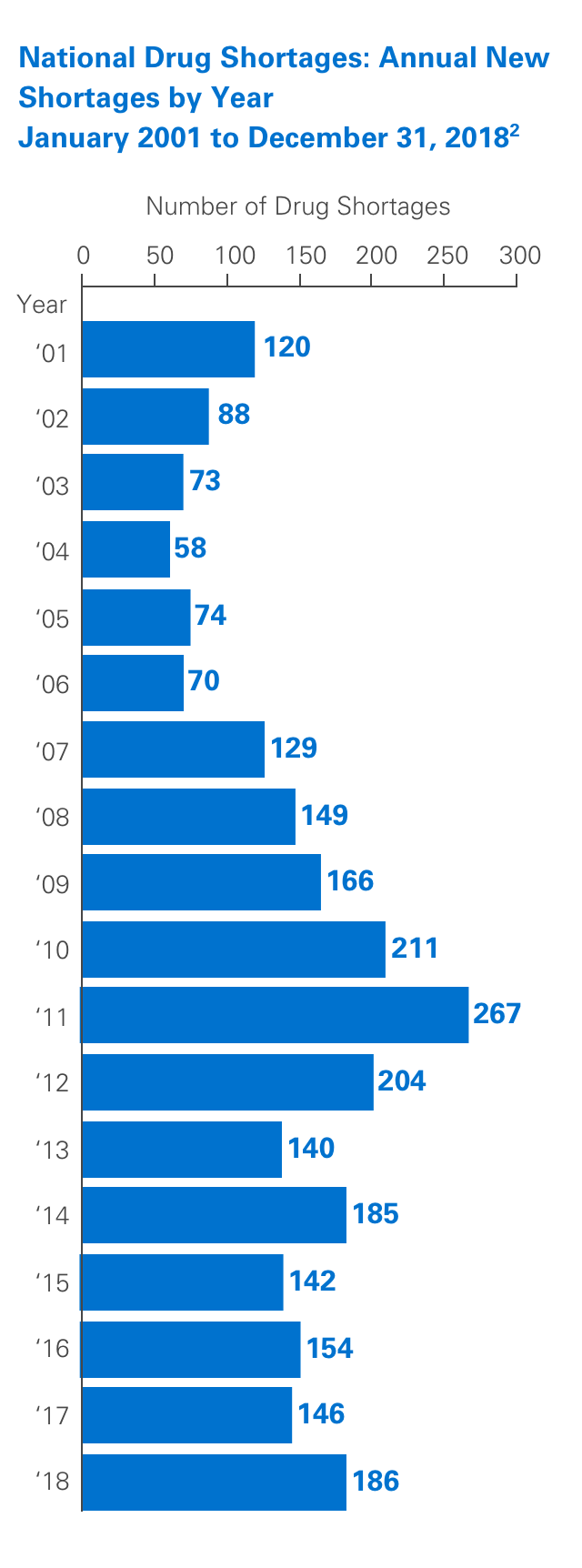
Unfortunately, drug shortages are common and have been the subject of numerous articles in recent years. Due to their highly specialized manufacturing process, sterile injectables make up a large percentage of these shortages. The number of new shortages has decreased overall since 2011.1,2
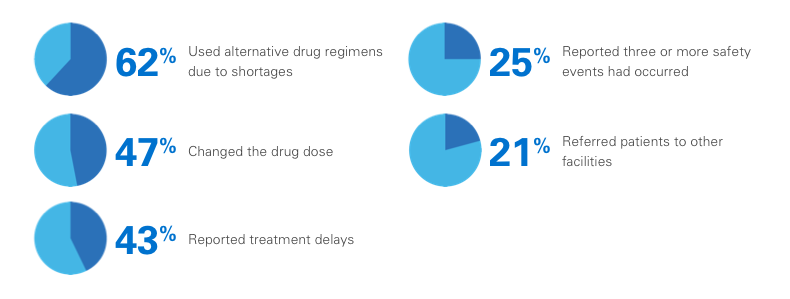
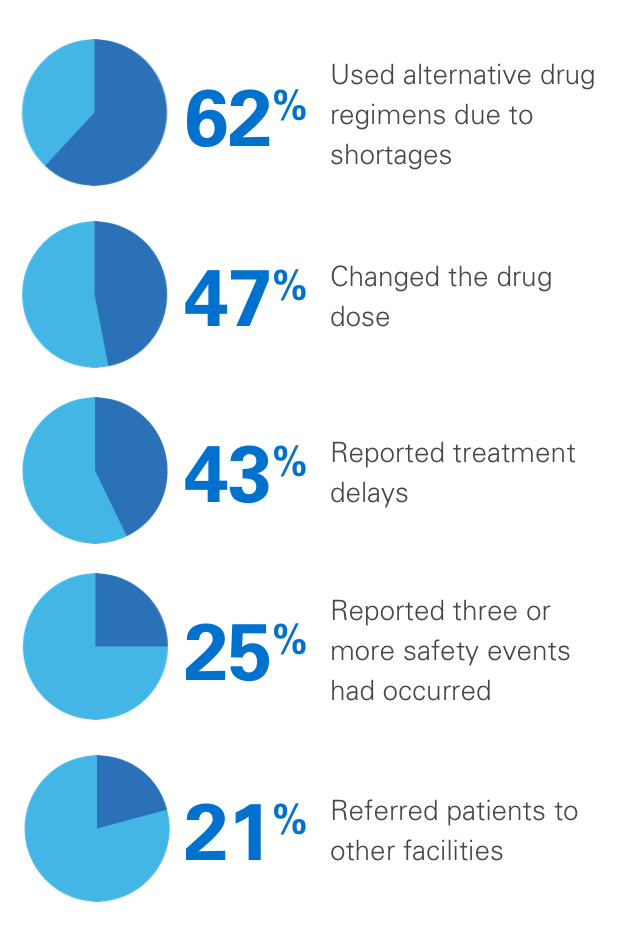
In one survey, 99% of pharmacy directors reported at least one injectable oncologic drug shortage during the previous year, with the following consequences:3
A 2010 survey of 353 pharmacy directors conducted by the American Society of Health-System Pharmacists (ASHP) and clinicians at the University of Michigan Health System (UMHS) found that the time and labor their staff spends managing drug shortages was significant, and the information available to manage drug shortages was suboptimal.4
To avoid treatment delays and unplanned switching between biologics during the course of treatment, it is important to consider a manufacturer’s history of shortages and recalls related to quality concerns, and evaluate its capability to maintain adequate production and stock to support demand. It is also important to consider the robustness of the manufacturer’s supply chain when evaluating biosimilars.5
References: 1. United States Government Accountability Office. Drug shortages. GAO-16-595. July 2016. www.gao.gov/assets/680/678281.pdf. Accessed October 29, 2019. 2. American Society of Health-System Pharmacists (ASHP). Drug shortages statistics. www.ashp.org/Drug-Shortages/Shortage-Resources/Drug-Shortages-Statistics. Accessed October 29, 2019. 3. Goldsack JC, Reilly C, Bush C, et al. Impact of shortages of injectable oncology drugs on patient care. Am J Health Syst Pharm. 2014;71:571-578. 4. Kaakeh R, Sweet BV, Reilly C, et al. Impact of drug shortages on U.S. health systems. Am J Health Syst Pharm. 2011;68:1811-1819. 5. Camacho LH, Frost CP, Abella E, et al. Biosimilars 101: considerations for U.S. oncologists in clinical practice. Cancer Med. 2014;3:889-899.