CLEAR
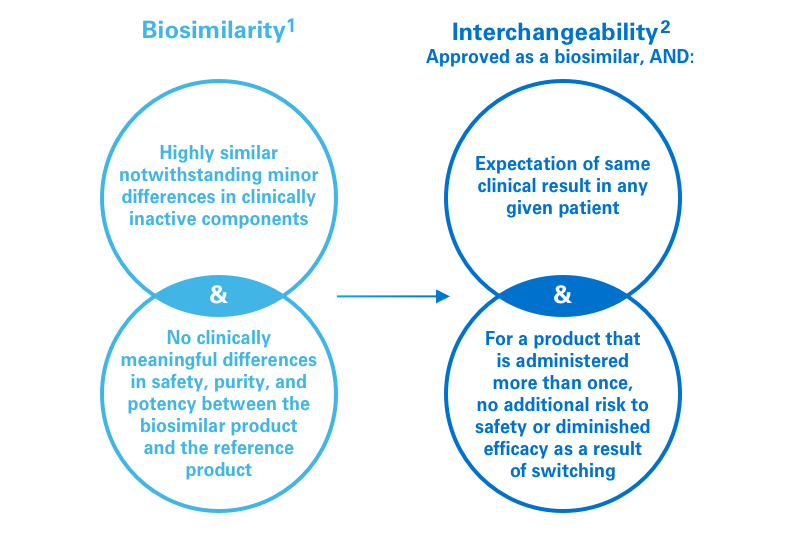
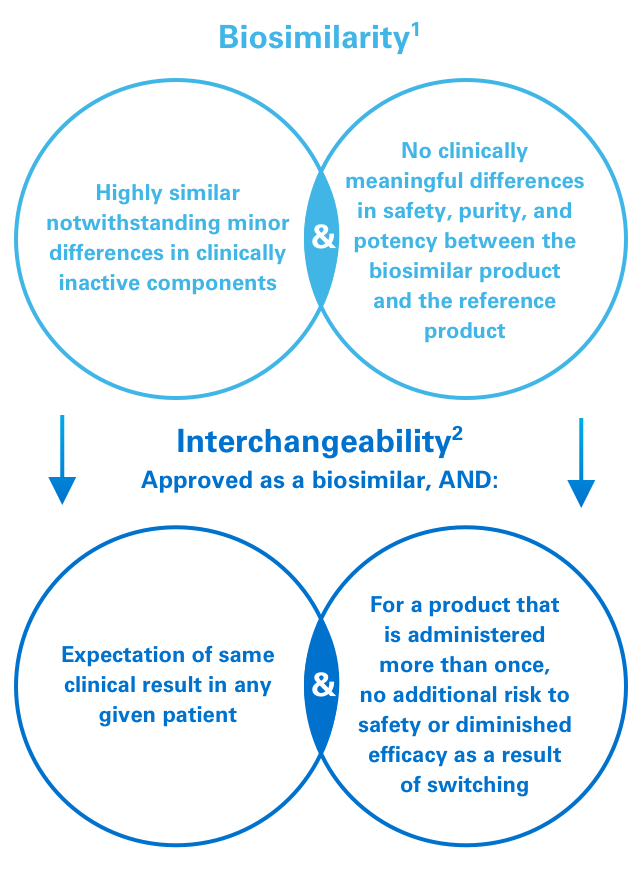
References: 1. US Food and Drug Administration. Guidance for Industry: Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. www.fda.gov/downloads/drugs/guidances/ucm291128.pdf. Published April 2015. Accessed January 10, 2019. 2. US Food and Drug Administration. Considerations in Demonstrating Interchangeability With a Reference Product. www.fda.gov.downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm537135.pdf. Accessed January 10, 2019.