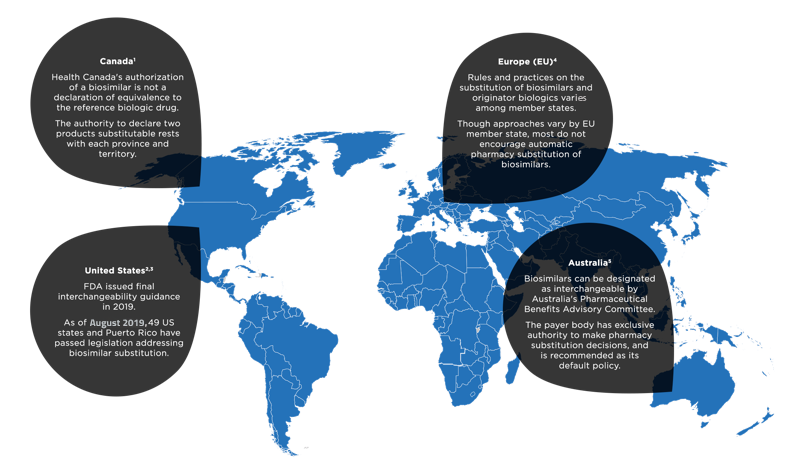
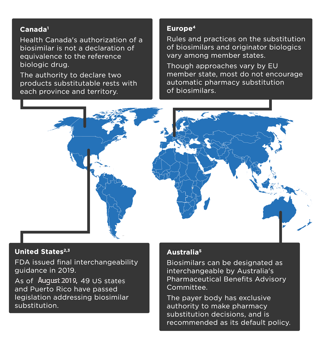
References: 1. Health Canada. Fact sheet: biosimilars. www.canada.ca/en/health-canada/services/drugs-health-products/biologics-radiopharmaceuticals-genetic-therapies/applications-submissions/guidance-documents/fact-sheet-biosimilars.html. Accessed October 29, 2019. 2. US Food and Drug Administration. Guidance for industry: considerations in demonstrating interchangeability with a reference product. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-demonstrating-interchangeability-reference-product-guidance-industry. Accessed October 29, 2019. 3. National Conference of State Legislatures. State laws and legislation related to biologic medications and substitution of biosimilars. www.ncsl.org/research/health/state-laws-and-legislation-related-to-biologic-medications-and-substitution-of-biosimilars.aspx. Accessed October 29, 2019. 4. Generics and Biosimilars Initiative. Biosimilar substitution in Europe. www.gabionline.net/Reports/Biosimilar-substitution-in-Europe. Accessed October 29, 2019. 5. Generics and Biosimilars Initiative. Interchangeability of biosimilars around the world. www.gabionline.net/Reports/Interchangeability-of-biosimilars-around-the-world. Accessed October 29, 2019.