At Amgen, we excel at the highly specialized, iterative process of developing monoclonal antibodies in vitro, scaling up the optimal cell line, time and again, in large-scale bioreactors, and checking and rechecking for batch-to-batch consistency.4,5
End-to-end biologics experts: our process4
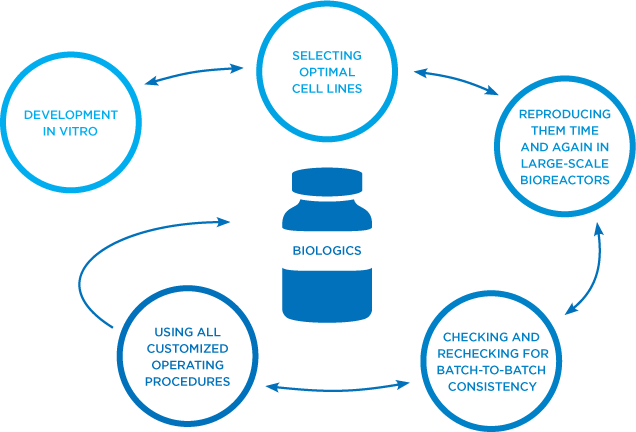
All of the complexities of manufacturing innovator biologics apply to biosimilar medicines as well. But biosimilar creation also has its own set of intricacies.
References: 1. Kozlowski S. US FDA perspectives on biosimilar biological products. Presented at: 2014 Biotechnology Technology Summit; June 13, 2014; Rockville, MD. www.ibbr.umd.edu/sites/default/files/public_page/Kozlowski%20-%20Biomanufacturing%20Summit.pdf. Accessed October 29, 2019. 2. US Food and Drug Administration. Guidance for industry: quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product. www.fda.gov/downloads/drugs/guidances/ucm291134.pdf. Accessed October 29, 2019. 3. US Food and Drug Administration. Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product. www.fda.gov/downloads/drugs/guidances/ucm291128.pdf. Accessed October 29, 2019. 4. Desanvicente-Celis Z, Gomez-Lopez A, Anaya JM. Similar biotherapeutic products: overview and reflections. Immunotherapy. 2012;4:1841-1857. 5. Ramanan S, Grampp G. Drift, evolution, and divergence in biologics and biosimilars manufacturing. BioDrugs. 2014;28:363-372. 6. Bee JS, Randolph TW, Carpenter JF, Bishop SM, Dimitrova MN. Effects of surfaces and leachables on the stability of biopharmaceuticals. J Pharma Sci. 2011;100:4158-4170. 7. Conner J, Wuchterl D, Lopez M, et al. The biomanufacturing of biotechnology products. In: Shimasaki C, ed. Biotechnology Entrepreneurship: Starting, Managing, and Leading Biotech Companies. Waltham, MA: Academic Press; 2014:351-385. 8. Blauvelt A, Cohen AD, Puig L, Vender R, van der Walt J, Wu JJ. Biosimilars for psoriasis: preclinical analytical assessment to determine similarity. Br J Dermatol. 2016;174:282-286. 9. Lybecker KM. The biologics revolution in the production of drugs. Fraser Institute. www.fraserinstitute.org/studies/biologics-revolution-in-the-production-of-drugs. Accessed October 29, 2019. 10. Dranitsaris G, Amir E, Dorward K. Biosimilars of biological drug therapies. Drugs. 2011;71:1527-1536. 11. Liu HF, Ma J, Winter C, Bayer R. Recovery and purification process development for monoclonal antibody production. mAbs. 2010;2:480-499. 12. Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol. 2008;19:411-419. 13. Roger SD. Biosimilars: how similar or dissimilar are they? Nephrology. 2006;11:341-346. 14. Hesse F, Wagner R. Developments and improvements in the manufacturing of human therapeutics with mammalian cell cultures. Trends Biotechnol. 2000;18:173-180.